Herborium, HBRM, Profile, Summary

Herborium, HBRM, Profile, Summary
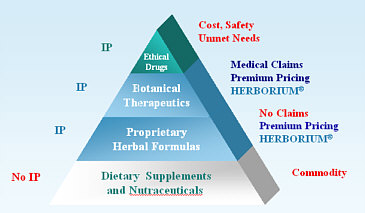
Herborium Group (HBRM) is a botanical therapeutics® company, develops, licenses and markets proprietary botanical based medicinal products to consumers and healthcare professionals.
Herborium develops its own formulations based on a herbaceutical science and other natural health resources including the traditional Chinese and herbal medicines from Peruvian Indians. These traditional botanical therapeutics are manufactured in technically advanced facilities strictly adhering to the FDA guidelines. The company's products are sold in The United States and Europe and, the company is planning to expand globally.
AcnEase®
Enhancing Beauty Botanically
The Company’s flagship product is AcnEase®, (www.acnease.com) a proprietary, all botanical acne treatment which has also been shown to improve conditions associated with Rosacea. The safety and efficacy of AcnEase has been validated by clinical testing. The results from these clinical studies demonstrated 96% improvement in both men and women with acne.
Over 60 million people in US and another 70-80 million in the Europe and Canada are estimated to suffer from acne (75% of individuals will have acne sometime in their life). The average age of individuals affected by acne increased during a last decade from 20 to 26 years old making adult acne one of a more prevailing health concerns.
The existing alternatives in the market for acne are limited to topical agents which treat existing acne, systemic antibiotics, and retinoic acid based products associated with severe side effects. A distinguishing characteristic of AcnEase aside from its remarkable safety profile is the products ability to prevent acne pimples from forming.
Lasting Pleasure for Women
More Power for Men!
A Natural Alternative for Female Sexual Dysfunction
Herborium’s Botanical Therapeutics® for Women (Lasting Pleasure) and Men (Lasting Power meet a growing interest in natural, safe sexual health products. Lasting Pleasure addresses the high demand for a “Viagra-like” product for women.
For female sexual dissatisfaction/dysfunction there currently are no systemic products available. The sole alternatives are topical agents and lubricants which have no impact on libido or female physical function.
The market for pharmaceutical grade sexual health products is over $3.2 billion worldwide with Viagra (Pfizer), Cialis (Eli Lilly) and Levitra (Bayer) leading market share. The market for natural sexual health products is estimated to be over $1.5 billion and growing. The rising costs of pharmaceutical grade sexual health drugs together with some safety concerns will drive consumers to look for alternatives in the market for natural sexual health products.
Key Strengths
> Proof of Concept through Revenues Generation. Significant and consistent sales and profit growth
> Proven ability to reduce time and therefore cost to market
> Proven management team with considerable experience working with early stage companies, digital and social marketing, healthcare products, drug development, clinical studies, regulatory compliance and international business.
> Regulatory know-how in the US and EU
> Access to a rich pipeline of qualified medicinal compounds and herbal medicines
> High level know-how in clinical research and pharmacological science
> Access to quality and novel formulation know-how, FDA, GMP, ICH and EMEA compliant manufacturing capabilities and competencies.
> Mitigation of investor risk, market entry risk, and therapeutic candidate risk based on initial data and screening performed in US, EU and Asia
> Strong Big Pharma connection in the USA and EU for co-licensing or licensing and co-marketing opportunities
> High Entry Barrier for competitors defined by the complex matrix of (Herborium’s) expertise indispensable to successfully managing the growing complexity of regulatory compliance, product development and market opportunities.
> Consumer and healthcare providers recent trends towards natural, organic, safer, plant-based medicine
> Healthcare economics stimulates search for new less expensive treatment options and encourages big Pharma and Insurance companies to look for consumer products and novel cost effective treatment options including Botanical Therapeutics®
Sources: The Company, OxBridge Research, OTCKING, DailyStockDeals, OTCstockIQ
Don't miss the NEXT premium Alert! Sign-up, Get Alerts,MakeMoney!®
Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
If you would like your company featured or want to learn more, please don't hesitate to contact the Editor. editor [@] OTCKING.com
ACNE,Adult Acne, Teen Acne, Botanical Acne medications, Natural Acne Medications, Lasting Pleasure,Lasting Love, Lasting erection, Lasting Power, Hard Erection, Dry Vagina, Wet Vagina, Libido, sex thoughts, Easy Arousal. HBRM. Herborium.
Aethlon Medical, AEMD, Profile, Summary
Aethlon Medical, AEMD, Profile, Summary

Aethlon Medical (AEMD) is developing innovative medical devices to address unmet medical needs in cancer, infectious disease, and other life-threatening conditions. Aethlon’s ADAPT™ platform provides the technology foundation for a new class of therapeutics that target the selective removal of disease enabling particles from the entire circulatory system. The Aethlon ADAPT™ product pipeline includes the Hemopurifier®, a first-in-class medical device with broad-spectrum capabilities against exosomes that contribute to the progression of cancer and infectious viral pathogens such as HIV and Hepatitis C.
HER2 Protein and Breast Cancer
Breast cancer is the most common form of cancer in women, accounting for 20% of cancer deaths with approximately 180,000 women diagnosed annually in the United States (Merrill, R. M., and A. Sloan. 2011. Risk-adjusted female breast cancer incidence rates in the United States. Cancer Epidemiol.). Over-expression of the human epidermal growth factor receptor 2 (HER2; ErbB2) gene occurs in approximately 25% of breast cancers and has been linked to poor prognosis (Slamon, D. J., G. M. Clark, S. G. Wong, W. J. Levin, A. Ullrich, and W. L. McGuire. 1987. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177-182). HER2 is a receptor tyrosine kinase, member of the EGF receptor family, which possesses proliferative and anti-apoptotic activities.
The standard of treatment for women diagnosed with HER2+ breast cancer includes the humanized monoclonal antibody trastuzumab (marketed as Herceptin®) directed against the extracellular domain of HER2. Trastuzumab binding to HER2 mediates direct growthinhibition of tumor cells and induces antibody-dependent cell cytotoxicity (ADCC), a major anti-cancer mechanism by which natural killer (NK) cells lyse antibody-coated tumor cells (Nahta, R., D. Yu, M. C. Hung, G. N. Hortobagyi, and F. J. Esteva. 2006. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol 3:269-280).
In patients with metastatic breast cancer undergoing chemotherapy, treatment with trastuzumab augments overall response rates and increases median survival time by 25% (Baselga, J. 2001. Clinical trials of Herceptin® (trastuzumab). Eur J Cancer 37 Suppl 1:18-24). However, response rates to trastuzumab range from 12% to 34% with median duration of 9 months due to development of resistance. Despite co-treatment with other HER2 targeting therapies (e.g. the tyrosine kinase inhibitor lapatinib) and chemotherapy, patients with metastatic breast cancer experience a limited duration of benefit; therefore, novel treatment strategies that act synergistically or overcome drug resistance are urgently needed (Sachdev, J. C., and M. Jahanzeb. 2011. Blockade of the HER Family of Receptors in the Treatment of HER2-Positive Metastatic Breast Cancer.
The Evolution of Therapeutic Filtration to Improve Hepatitis-C Virus (HCV) Treatment Outcomes
Therapeutic filtration of HCV from the entire circulatory system with a medical device has been clinically demonstrated to improve treatment outcomes when administered at the outset of standard of care (SOC) drug therapy. This summary overview, which includes an introduction to the Aethlon Hemopurifier®, is for informational purposes only.
About HCV Infection
The U.S. National Institutes of Health (NIH) reports an estimated 180 million people worldwide are afflicted with chronic HCV infection. Of people infected, 55 to 85% of will develop chronic infection, and 75% of those will develop chronic liver disease. Standard of care (SOC) drug therapy is represented by a two drug combination of interferon and ribavirin. The primary goal of SOC drug therapy is the achievement of a sustained virologic response (SVR), defined as undetectable viral load six months after completion of SOC drug therapy. According to the NIH, 15-25% of HCV infected patients will recover
completely.
SOC Drug Therapy Outcomes
•The largest study of HCV infected individuals pursuing 48-week SOC drug
therapy was a study of 4,469 genotype 1 patients published by McHutchison in The New England Journal of Medicine in August of 2009.
•31% (1,399 of 4,469) of patients screened for participation were excluded from the study.
•54% (1,654 of 3,070) of treated patients completed the SOC treatment regimen.
•39.6% (1,215 of 3,070) of treated patients who completed SOC therapy
achieved a SVR.
•27% (1,215 of 4,469) of the total patients screened for study participation
achieved a SVR.
•No data recorded for patients who may have relapsed after achieving a SVR.
The Hemopurifier®
The first medical device to selectively remove HCV and related immunosuppressive proteins from circulation. A Study of the Hemopurifier® + SOC Drug Therapy is Now Underway
•Patient enrollment has been initiated at the Medanta Medicity Institute in India - www.medanta.org
•Additional details can be accessed from the Clinical Trials Registry - India (CTRI) link: http://ctri.nic.in/clinicaltrials/index.jsp
•Up to 30 patients / up to 6 treatments in first 3 days of SOC
•Early clinical endpoints include:
•Immediate Virologic Response (IVR)
•Rapid Virologic Response (RVR)
•Early Virologic Response (EVR)
The Hemopurifier® Other Selected Quick Facts
•70 human treatment experiences administered to date
•An Investigation Device Exemption (IDE) to initiate U.S. studies has been filed with FDA
•Substantial viral load reductions have also been observed in a human HIV study performed in the absence of antiviral drugs
•GMP manufacturing has been established in an FDA approved facility
•The Hemopurifier® has proven broad-spectrum capabilities against viral bioterror and pandemic threats
•The device has been discovered to capture tumor-secreted exosomes known to suppress the immune system of cancer patients
Sources: The Company, OxBridge Research, OTCKING, DailyStockDeals, OTCstockIQ
Don't miss the NEXT premium Alert! Sign-up, Get Alerts,MakeMoney!®
Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
If you would like your company featured or want to learn more, please don't hesitate to contact the Editor. editor [@] OxBridgeResearch.com
ALS, Hepatitis C, Traumatic Brain Injury, Blood Sepsis,breast cancer,HIV,HPV,HCV,SOC, dialysis,DOD,UNT,USPTO, NFL,SOCCOR,MILITARY,COMBAT MISSIONS,PTSD, Ebola
Premier Biomedical, BIEI, Profile, Summary

Premier Biomedical | BIEI | Profile | Summary
Premier Biomedical (BIEI) is a research-based company that has acquired exclusive licenses for patent-pending medications and medical procedures to develop cures for a significant number of the most debilitating and often fatal illnesses: ALS, Traumatic Brain Injury, Multiple Sclerosis, Clinical Depression, Alzheimer’s Disease, Blood Sepsis, and Cancer. The company is in the process of developing targeted medicines and procedures and will prove out their superior efficacy in addressing these diseases and others through laboratory, hospital, and actual patient applications. At the anticipated successful conclusion of the clinical trials, contact will be established with the leading worldwide pharmaceutical firms to establish the right to market and distribute Premier Biomedical’s technology products and procedures
INVESTMENT HIGHLIGHTS
· The Company’s proprietary Sequential Dialysis Technique and patented candidate drug Feldetrex are expected to provide superior efficacy versus existing medications in treating a large number of the most fatal diseases
o Alzheimer’s Disease, Multiple Sclerosis, ALS, Fibromyalgia, Traumatic Brain Injury, Blood Sepsis, and Cancer
· The Company has established two outstanding research partnerships with the University of Texas, El Paso (UTEP) and the Department of Defense
o Leverage the substantial infrastructure and resourced capacity of these organizations to perform experimentation and to engage in product development in an inexpensive and efficient manner
o Positive results in animal testing for the Sequential Dialysis Technique treatment of cancer
o Initiation of two clinical trials including a trial for Feldetrex and a trial of the Sequential Dialysis Technique
EXECUTIVE SUMMARY
· Premier Biomedical is a research-based company that intends to discover and develop medical treatments targeting the treatment of: Alzheimer’s Disease, Multiple Sclerosis, ALS, Fibromyalgia, Traumatic Brain Injury, Blood Sepsis, and Cancer
· The Company’s proprietary Sequential Dialysis Technique is a methodology that physically removes the pathophysiologic basis of the disease, eliminating it without dangerous side effects
o Superior to current treatments which eliminate the presence of most illnesses but often with catastrophic or even fatal side effects
o Targets cancer, Alzheimer’s disease, Multiple Sclerosis, ALS, blood sepsis, and Traumatic Brain Injury – collectively over $700 billion market opportunity
· Developed a proprietary drug candidate Feldetrex as a potential treatment for Multiple Sclerosis, Fibromyalgia, and Traumatic Brain Injury
o Expected to deliver significant relief to patients, while presenting fewer side effects than other alternate medications
o The Company was recently granted two US patents for this drug candidate
o The annual market size of all proposed market segments for Feldetrex is $16 billion
· The Company has established two outstanding research partnerships with the University of Texas, El Paso (UTEP) and the Department of Defense
o Leverage the substantial infrastructure and resourced capacity of these organizations to perform experimentation and to engage in product development in an inexpensive and efficient manner
o Positive results in animal testing for the Sequential Dialysis Technique treatment of cancer
o Initiation of two clinical trials including a trial for Feldetrex and a trial of the Sequential Dialysis Technique
Sources: The Company, OxBridge Research, OTCKING, DailyStockDeals, OTCstockIQ
Don't miss the NEXT premium Alert! Sign-up, Get Alerts,MakeMoney!®
Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
If you would like your company featured or want to learn more, please don't hesitate to contact the Editor. editor [@] OTCking.com
Axxess Pharma, AXXE, Profile, Summary
Axxess Pharma, AXXE, Profile, Summary

Axxess Pharma (AXXE) is a specialty Health Care Products Company dedicated to improving health and quality of life by offering select medicines, nutritional supplements and over the counter remedies all across the Americas. Axxess's goal is to bring additional products to the market and provide new, innovative options for better health spanning areas such as high cholesterol, blood pressure, acute pain as wells as nutritional supplements.
Axxess Pharma is dedicated to providing a wide range of products to improve the lives of patients and consumers worldwide.
Axxess Pharma intends to market these products with affordable pricing and widespread availability. The products are targeted to improve health-related conditions including several forms of iron deficiency, bone loss, rheumatoid arthritis, migraine headaches, urinary tract infections, infant cradle cap, joint pain and much more. Axxess Pharma will continue to tap the well of innovation to develop ever-more effective and affordable treatment options for the patients.
Axxess Pharma Signed an Exclusive World-Wide License to Market and Sell TapouT Brand Vitamins & Minerals Pain Relief and Muscle Recovery Products. http://shop.tapout.com/
Sources: The Company, OxBridge Research, OTCKING, DailyStockDeals, OTCstockIQ
Don't miss the NEXT premium Alert! Sign-up, Get Alerts,MakeMoney!®
Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
Want to get your company featured? or have questions/comments, please don't hesitate to contact the Editor. editor [@] OxBridgeResearch.com
- Dalton Industries, DALT, Profile, Summary
- Integrated Environmental Tech, IEVM, Profile, Summary
- Integrated Environmental Tech, IEVM, Profile, Summary
- KiOR, KIOR, Profile, Summary
- ZaZa Energy , ZAZA, Profile, Summary
- The Alkaline Water Company, WTER, Profile
- Hemispherx Biopharma, HEB, Profile, Summary





 Feed Entries
Feed Entries
